Where’s the Beef? Cell-Cultured Meat Grown in a Lab Needs Regulation
By Jayne Roth
What will we find next on the grocery store shelf? A plethora of food and beverages beyond your imagination continue to be created thanks to advances in biotechnology and global supply chains. Food scientists and manufacturers are constantly developing cutting-edge products that tout being healthier and better for the environment, among other claims. Enter “cell-cultured” meat and seafood. How and who will regulate these novel foods to ensure they meet labeling requirements and are safe to eat, not adulterated or misbranded?
Within the industry, there are concerns about what to call these products – labels must provide information regarding their origin and method of production. Possibilities include cell-based meat, cell-cultured meat, cultivated meat, cultured meat, and lab-grown meat. A 2020 study conducted by William K. Hallman interviewed 3,186 adults and found that “cell-based” meat or seafood was the best option chosen by consumers. However, “cell-cultured” is more routinely used by the FDA and USDA at this time.
Cultured meat is produced by taking animal cells from a tissue biopsy and placing those cells into a controlled and sterile environment. Then a liquid growth medium; which is comprised of vitamins, proteins, sugars and amino acids, is added for anywhere from a couple of weeks to over a month. The finished product is harvested in a bioreactor when the desired amount of meat or seafood is grown. This has drawn the ire of traditional meat producers, specifically the US Cattlemen’s Association, who “welcome competition and consumer choices,” but are asking for a “level playing field for real beef products and meat analogues alike.” Alternatively, the North American Meat Institute’s position is that the cell-cultured products meet the US Department of Agriculture’s (USDA) definition of meat. The US Cattlemen’s Association provided a statement requesting that the definition of beef be limited to products from cattle-born, raised, and harvested in this traditional manner.
Currently, there are more than 40 companies working to create cell-cultured meat from traditionally animal and seafood-based products (ex: hamburger, steak, fish, duck, pork, and chicken). Financial support for cell-grown meat has come from eminent investors and entrepreneurs such as Bill Gates and Richard Branson. Blue Chip companies such as Tyson Foods and Cargill have also invested or bought small cell-cultured meat brands.
Regulatory Oversight
Regulatory oversight for processing and labeling of cell-cultured animal products is being approached cautiously by the US Food and Drug Administration (US FDA) and USDA’s Food Safety Inspection Service (FSIS). In March 2019, the two agencies signed a formal agreement and outlined which agency will oversee each piece of production, including labeling.
At this time, federal regulations have not changed or added to current legislation to address cell-cultured products. The US FDA indicated that they have “sufficient authority under the Federal Food, Drug and Cosmetic Act’s (FD&C Act) food safety provisions to regulate and ensure the safety of foods made from cultured animal cells”. In addition, cultured meat labels will be pre-approved by the FSIS as required in the Federal Meat Inspection Act (FMIA) and the Poultry Inspection Act (PPIA) which are already implemented.
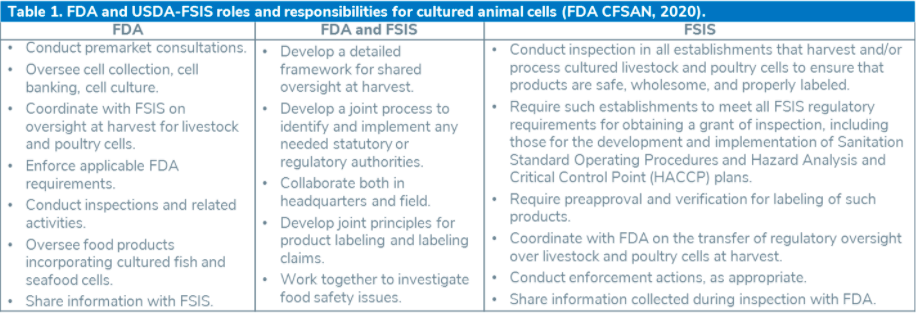
Table 1. below separates the responsibilities outlined in the agreement between FDA and USDA in regard to cultured animal cells:
There are no cell-cultured products available for purchase in the US today. Yet, with numerous large brands working toward the ultimate goal of retailing these products, the FDA is working towards issuing specific guidance for premarket consultation. FSIS is working closely with FDA on common principles for the labeling of cell-cultured products. FSIS will develop regulatory requirements for products made from cells of meat and poultry to ensure that the labeling is not misleading about the nature of its production, and intends to publish an Advance Notice of Proposed Rulemaking in the coming months.
Missouri is first to pass legislation regulating cell-cultured meats. Several other states have indicated they will follow Missouri’s lead, especially out of concern that cell-cultured foods will be referred to as “meat.” However, any legislation passed by states regarding labeling which is contrary to federal laws (FMIA or PPIA) will be preempted.
It is important that federal agencies and other supporting commodity organizations introduce this product into commerce mindfully. A lack of scientific understanding about lab-grown meat may cause a misperception and potential stigma with these products – similar to concerns about GMO foods, irradiated foods and antibiotics used in livestock.
About the Author
Jayne Roth is an independent Food Safety and public health consultant with a master’s degree in public health. She has worked with large companies, such as Amazon and Walmart, she has created assessments for facilities, and she has marketed various company’s private Food Handlers Courses to public health jurisdictions for approval. Her special interests include infectious disease control and food safety rule interpretations. She is well-versed in food safety regulatory compliance and regulatory structure of public health jurisdictions.

-
 FeaturedRisk management
The Cost of a Breach: What a Cyberattack Could Mean for Food Safety Recalls
FeaturedRisk management
The Cost of a Breach: What a Cyberattack Could Mean for Food Safety Recalls
-
 FeaturedRisk management
Securing the Food Chain: How ISO/IEC 27001 Strengthens Cybersecurity
FeaturedRisk management
Securing the Food Chain: How ISO/IEC 27001 Strengthens Cybersecurity
-
 FeaturedRisk management
Revolutionizing Food Safety Training: Breaking Out of the “Check-the-Box” Mentality
FeaturedRisk management
Revolutionizing Food Safety Training: Breaking Out of the “Check-the-Box” Mentality
-
 GFSI Standards
GFSI 2025: Building Trust, Tech-Forward Solutions, and Global Unity in Food Safety
GFSI Standards
GFSI 2025: Building Trust, Tech-Forward Solutions, and Global Unity in Food Safety
-
 FeaturedFood Safety
Integrated Pest Management: Strategies to Protect Your Brand’s Reputation
FeaturedFood Safety
Integrated Pest Management: Strategies to Protect Your Brand’s Reputation
-
 FeaturedFood Safety Culture & Training
No Open Door Policy: Challenges That Impact Pest Control in Food Processing Plants
FeaturedFood Safety Culture & Training
No Open Door Policy: Challenges That Impact Pest Control in Food Processing Plants