Three Things You Need to Know about Preventive Controls
Food Safety Modernisation Act (FSMA):
Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food
As part of the Food Safety Modernization Act (FSMA), the US Food and Drug Administration has issued several rules to further clarify the requirements of the regulations, one of which relates to Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food. The rule was developed after extensive consultation with numerous stakeholders.
Exemptions
The limitations and definitions of which facilities are covered by this rule are many and varied and take pages to define. A few examples of the exemptions include:
-
- Farms (to be discussed below) that engage in low-risk on-farm activities and are small or very small businesses
-
- Operations already subject to the seafood or juice HACCP requirements
-
- Operations that process low-acid canned foods
-
- Producers of dietary supplements, alcoholic beverages or cosmetics, all of which have their own separate regulations
-
- Facilities that store raw agricultural commodities (RACs) other than fruits or vegetables or that store already packaged foods.
Requirements for Non-Exempt Facilities
All other facilities are covered by the rule and therefore must establish and implement a food safety system. This requires a written food safety plan that covers
-
- hazard analysis
-
- preventive controls
-
- oversight and management:
-
- monitoring of the preventive controls
-
- corrective actions and corrections
- corrective actions and corrections
-
- verification that the above activities are scientifically valid, effective and are being carried out regularly
-
- oversight and management:
Definition of a Farm
Farms are not subject to this rule; instead they are subject to the Produce Safety Rule, which has expanded and clarified the definition of what constitutes a farm. There are two main types identified:
-
- a Primary Production Farm:
-
- Is under one management in one general area but not necessarily on the same piece or adjoining pieces of property
-
- Is dedicated to growing and harvesting crops, raising animals (including seafood) or any combination thereof
-
- May conduct some manufacturing or processing activities, such as dehydrating grapes to make raisins, as well as packaging and labelling
-
- a Primary Production Farm:
-
- A Secondary Activities Farm:
-
- Is not located on the Primary Production Farm but is harvesting, packing and/or holding RACs
-
- May or may not have the same owners as the Primary Production Farm
- May or may not have the same owners as the Primary Production Farm
-
- A Secondary Activities Farm:
This means that facilities that do off-farm packing are now included within the definition of a farm, as are companies that do nothing but harvest crops, and companies that merely hold or store RACs.
Supply Chain Programs
Another important area that is addressed by this rule is the supply chain. The aim is to make this process more flexible. As well, it establishes separate compliance dates so that a food facility does not have to comply before its suppliers do.
A facility must have a risk-based preventive assessment supply chain program for any ingredient or material that has been identified as needing one, unless it either institutes preventive measures to deal with the hazard itself or passes the ingredient on to a customer who will implement their own preventive measures.
Foods should be received from approved suppliers, which are defined as ones approved by the facility after an assessment of the hazard associated with that particular food and the track record of the entity that will be controlling that hazard. Of course, foods may be brought in temporarily from unapproved suppliers, but such ingredients are then subject to verification before they can be used.
Preventive controls are not required within the facility if the hazard will be controlled by a subsequent entity such as the final customer or another processor. However, the facility must disclose that this food has not been processed to control its associated risk (e.g. Salmonella or listeria) and must obtain written assurance that the subsequent entity will do so.
Another entity, such as a broker or a distributor, can conduct supplier verifications but the receiving facility must review and assess that entity’s documentation of the verification.
Current Good Manufacturing Processes (CGMPs)
The rule attempts to update and clarify CGMPs. As well, the rule no longer includes any non-binding provisions or guidelines. All provisions are now binding. These include:
-
- Education and training:
-
- All employees must be qualified to perform their assigned duties
-
- All employees managing risk as well as those implementing preventive controls must be qualified to do so either by training or experience
-
- Education and training:
-
- Allergen cross-contact: this has long been included in the FDA’s CGMPs but now they are included within the regulatory text.
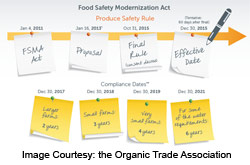
Compliance Dates
The rule was passed on September 17, 2015 and compliance is required from that date as follows:
-
- 3 years for very small businesses (averaging less than $1 million in sales and/or held inventory per year). Records to establish it as a very small business were due by January 1, 2016.
-
- 3 years for businesses subject to the Pasteurized Milk Ordinance
-
- 2 years for small businesses (fewer than 500 full-time equivalent employees)
-
- 1 year for all other businesses
Compliance dates for the requirements of the supply chain program:
-
- 2 years if the receiving facility is a small business and its supplier will not be subject to the human preventive controls rule or the produce safety rule
-
- 2 years or 6 months after the supplier is required to comply, whichever is later, if the receiving facility is a small business and its supplier will be subject to the human preventive controls rule or the produce safety rule
-
- 18 months if the receiving facility is not a small or very small business and its supplier will not be subject to the human preventive controls rule or the produce safety rule
-
- 6 months after the supplier is required to comply with the applicable rule if the receiving facility is not a small or very small business and its supplier will be subject to the human preventive controls rule or the produce safety rule.

-
 FeaturedRisk management
The Cost of a Breach: What a Cyberattack Could Mean for Food Safety Recalls
FeaturedRisk management
The Cost of a Breach: What a Cyberattack Could Mean for Food Safety Recalls
-
 FeaturedRisk management
Securing the Food Chain: How ISO/IEC 27001 Strengthens Cybersecurity
FeaturedRisk management
Securing the Food Chain: How ISO/IEC 27001 Strengthens Cybersecurity
-
 FeaturedRisk management
Revolutionizing Food Safety Training: Breaking Out of the “Check-the-Box” Mentality
FeaturedRisk management
Revolutionizing Food Safety Training: Breaking Out of the “Check-the-Box” Mentality
-
 GFSI Standards
GFSI 2025: Building Trust, Tech-Forward Solutions, and Global Unity in Food Safety
GFSI Standards
GFSI 2025: Building Trust, Tech-Forward Solutions, and Global Unity in Food Safety
-
 FeaturedFood Safety
Integrated Pest Management: Strategies to Protect Your Brand’s Reputation
FeaturedFood Safety
Integrated Pest Management: Strategies to Protect Your Brand’s Reputation
-
 FeaturedFood Safety Culture & Training
No Open Door Policy: Challenges That Impact Pest Control in Food Processing Plants
FeaturedFood Safety Culture & Training
No Open Door Policy: Challenges That Impact Pest Control in Food Processing Plants