Phage-based Bioactive Paper a Safe Breakthrough for Detecting Bacterial Pathogens in Food
By Hany Anany and Mansel Griffiths
Despite the adoption of various preventive management systems such as HACCP, and the introduction of new technologies, outbreaks of foodborne illness continue to occur and they are accompanied by the burgeoning appearance of antibiotic-resistant strains of bacterial pathogens. Thus, our armory to combat these bacterial infections has to be expanded.
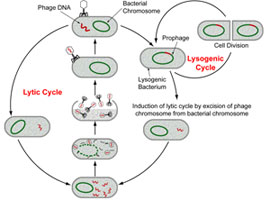
Bacteriophages (phages) have been deployed as a promising technology to control the growth of various foodborne bacteria since their discovery early in the 20th Century. Lytic phages are viruses—mostly from 20 to 200 nanometres in diameter—that can infect and replicate within bacteria in a strain-specific manner. When a key number of phages accumulate in the cell, they will lyse (dissolve) the bacterial cells.
The key to their specificity are the proteins in their tail fibres which recognize receptors on the surfaces of bacterial cell walls.
The potential for the development of phage-based methods for detecting and controlling pathogenic bacteria is enormous. Phages exist in many different environments and are deemed to have no harmful effect on humans. Actually, regulatory approval for their application in the food industry has been granted in Europe, the US and Canada. Phages can be added at different stages of food production and processing to control the growth of bacterial pathogens without changing the colour, texture, taste or flavor of the food. Most common implementations involve dipping the foodstuff in a suspension of phages and/or spraying phages on the food’s surface. However, these methods may not be ideal, as they could be wasteful, lead to potential development of phage resistant strains and ultimately result in the inactivation of the phage.
Furthermore, although the direct addition of free “viruses” to ready-to-eat foods, is safe, the notion may be unacceptable to consumers. One strategy to avoid these problems is to immobilize bacteriophages on food contact surfaces, such as packaging material. This will avoid phage wastage, ensure direct contact of the phage with the food surface, and alleviate consumers’ concerns about eating “viruses” in their food. In addition, phage-based paper strips can be used to specifically capture and detect bacterial pathogens in food samples.
Our research group has identified ways to immobilize phages on paper, which can then be used for both detection and biocontrol. In collaboration with a research team from McMaster University and funding from the NSERC Sentinel Bioactive Paper Network, we’ve been able to develop ways to maintain phage stability after immobilization on paper by printing or coating. For immobilized phages to work, it is crucial to attach the phages to the support matrix in the right orientation; that is, with their tail fibers accessible to specifically capture the target bacterium. As the tail fibres of phages are positively charged and their heads are negatively charged, electrostatic interactions can be used to simplify oriented immobilization.
We have also developed a bioink formula that stabilizes the phages during inkjet piezoelectric printing onto cationic paper. This process allows the attachment of phage heads to the paper surface, leaving the tail fibers free to recognize receptors on the bacterial cell surface.
Dipsticks printed with phages can capture foodborne pathogens such as E. coli O157:H7, E. coli O45 and Salmonella Newport from food. After allowing a short time for the phage to infect their host, the progeny phages produced can be detected by nucleic acid amplification techniques such as qPCR. Using this technique, as few as 10 cells/g of food can be detected within eight hours. Using immobilized phages makes it easy to remove the phages that have not infected the bacterium so that only progeny are detected. This detection method is rapid, specific, sensitive, cost-effective and can be applied to a broad range of foodborne pathogens. We have also demonstrated that Listeria monocytogenes-specific phages coated onto butcher’s paper can control the growth of Listeria monocytogenes on ready-to-eat meats.
This is an exciting field and we’re finding that there are many ways in which immobilized phages can be used to enhance food safety. More will undoubtedly develop as time goes on, and it’s to be hoped that the cause of food safety in general—and consumer health in particular—will be the beneficiaries.
About the Author
Dr. Mansel Griffiths has recently retired from the University of Guelph where he held an NSERC=DFO Senior Industrial Research Chair and was the founding Director of the Canadian Research Institute for Food Safety. He has authored over 350 peer reviewed for journal articles and is an ISI Highly Cited author. He serves as editor of numerous journals and on several government and industry expert advisory groups on food safety. He continues his interest in research on the control and detection of foodborne pathogens.
Dr. Hany Anany is currently a Research Associate at the Canadian Research Institute for Food Safety, Food Science Department of University of Guelph. In 2010, he completed his PhD in Food Science from the same university. Dr. Anany’s current research areas involve isolation and genome characterization of bacteriophages for taxonomical and application purposes. He is investigating the use of non-immobilized and immobilized bacteriophages to control and detect various foodborne in food. He has published 15 peer reviewed scientific journal articles and 3 book chapters.
To have more articles like this emailed to your inbox, become a GFSR Member today!

-
 FeaturedRisk management
The Cost of a Breach: What a Cyberattack Could Mean for Food Safety Recalls
FeaturedRisk management
The Cost of a Breach: What a Cyberattack Could Mean for Food Safety Recalls
-
 FeaturedRisk management
Securing the Food Chain: How ISO/IEC 27001 Strengthens Cybersecurity
FeaturedRisk management
Securing the Food Chain: How ISO/IEC 27001 Strengthens Cybersecurity
-
 FeaturedRisk management
Revolutionizing Food Safety Training: Breaking Out of the “Check-the-Box” Mentality
FeaturedRisk management
Revolutionizing Food Safety Training: Breaking Out of the “Check-the-Box” Mentality
-
 GFSI Standards
GFSI 2025: Building Trust, Tech-Forward Solutions, and Global Unity in Food Safety
GFSI Standards
GFSI 2025: Building Trust, Tech-Forward Solutions, and Global Unity in Food Safety
-
 FeaturedFood Safety
Integrated Pest Management: Strategies to Protect Your Brand’s Reputation
FeaturedFood Safety
Integrated Pest Management: Strategies to Protect Your Brand’s Reputation
-
 FeaturedFood Safety Culture & Training
No Open Door Policy: Challenges That Impact Pest Control in Food Processing Plants
FeaturedFood Safety Culture & Training
No Open Door Policy: Challenges That Impact Pest Control in Food Processing Plants