Are Pathogens Sneaking Past Your Sampling Method? Automate to Minimize Your Business Risk
By Martha Stone
Professionals in the food safety arena know that the FDA’s new approach for FSMA is prevention of, rather than reaction to, foodborne illness outbreaks. But a prevention-based approach requires the collection of data to identify any potential hazards that may be present in the food production process before the food reaches the marketplace. The challenge is that a business’s pathogenic organism detection data is only as good as the sample tested.
The FDA states that if samples are improperly collected and mishandled, or are not representative of the sample lot, the laboratory results will be meaningless. It further outlines three food categories that specify how many samples should be collected in a sampling plan. Most food companies follow sampling plans of n=60, n=30, or even n=15, where food samples of 25g each are taken based on Food Category I, II, or III, respectively. But are these truly representative samples? Will they ensure an adulterant is found if present? Far too often, the accuracy of lot testing proves to be inadequate to ensure safe products.
A business’s pathogenic organism detection data is only as good as the sample tested.
Pathogen Contamination
The food industry’s most common sample collection method is “grab sampling,” which consists of a manual grab from a process line. The common practice is to take multiple grab samples over a period of time to make a composite sample. The main advantage of this method is the low cost. However, this method can lead to imprecision errors, sample bias, and even potential product contamination. Additionally, pathogens typically exist in small, localized pockets of contamination within the bulk food product, where limited grab sampling is likely to miss the contamination. If the distribution of the target adulterant is non-homogeneous, grab sampling simply cannot produce a truly representative food sample.
The International Commission on Microbiological Specifications for Foods (ICMSF) develops guidelines of recommended microbiological sampling and testing methods that are statistically relevant. Two-class attribute sampling plans are commonly used throughout the food industry, and they are based on classifying each sample unit, taken through grab sampling, as acceptable or unacceptable based on their microbiological limit. However, because of the nature of pathogen contamination in food, there is a high probability of missing the contamination using these attribute sampling plans. The chart below is a clear example of this. In a sampling plan based on an n=60 analytical units collected using grab sampling, and a contamination level of only 1%, the probability of missing the pathogen contamination is 55%.
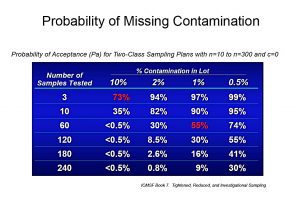
This percentage is a significant risk, considering the implications of a food recall. Nonetheless, there are new technologies that allow sampling to be performed on a “near continuous” level and are far more representative than other sampling methods. When using continuous sampling technologies, a small amount of the food product is collected from each unit during production, and a composite is accrued over time. This continuous method of sampling provides a more statistically representative sample from which to test. Other advantages of this technology are the elimination of imprecision due to the use of automated equipment, a better control over sample bias as samples are more statistically representative, and the prevention of contamination due to manual grab sampling. On the downside, there is a capital investment to consider when switching to automated continuous sampling technologies. However, the cost of a food recall would be much higher.
Grab sampling, by nature, invites human error. Automated continuous sampling, on the other hand, ensures equipment-based consistency. And there is the issue of the representativeness of a sample to consider, and its accuracy. Obtaining actionable data for a food safety system requires a fully comprehensive sampling technique that will help reduce the risk of a potential hazard. Lives are at stake. And so is your brand.
About the Author
Dr. Martha Maradiaga-Stone is a Food Safety Microbiologist (Ph.D., Texas Tech University) working as the Food Safety Manager for Hollison, LLC. Hollison is a high-tech food safety company focused on the detection of pathogens using novel sampling solutions as well as protection from pathogens in animal and human food and their environments, using next level probiotics. Dr. Maradiaga-Stone leads a team that is committed to finding innovative and streamlined ways to enhancing the safety of food.

-
 FeaturedRisk management
The Cost of a Breach: What a Cyberattack Could Mean for Food Safety Recalls
FeaturedRisk management
The Cost of a Breach: What a Cyberattack Could Mean for Food Safety Recalls
-
 FeaturedRisk management
Securing the Food Chain: How ISO/IEC 27001 Strengthens Cybersecurity
FeaturedRisk management
Securing the Food Chain: How ISO/IEC 27001 Strengthens Cybersecurity
-
 FeaturedRisk management
Revolutionizing Food Safety Training: Breaking Out of the “Check-the-Box” Mentality
FeaturedRisk management
Revolutionizing Food Safety Training: Breaking Out of the “Check-the-Box” Mentality
-
 GFSI Standards
GFSI 2025: Building Trust, Tech-Forward Solutions, and Global Unity in Food Safety
GFSI Standards
GFSI 2025: Building Trust, Tech-Forward Solutions, and Global Unity in Food Safety
-
 FeaturedFood Safety
Integrated Pest Management: Strategies to Protect Your Brand’s Reputation
FeaturedFood Safety
Integrated Pest Management: Strategies to Protect Your Brand’s Reputation
-
 FeaturedFood Safety Culture & Training
No Open Door Policy: Challenges That Impact Pest Control in Food Processing Plants
FeaturedFood Safety Culture & Training
No Open Door Policy: Challenges That Impact Pest Control in Food Processing Plants




